Food security in the prevailing uncertain climatic and economic conditions can only be guaranteed by deliberate actions toward maximizing production, especially in stress-prone environments. The main priority of the CGIAR and NARS breeding programs is to enhance genetic gain in crops through the assessment of seed varieties with drought-resilient, nutritional, and yield traits. This is achieved by leveraging data-driven approaches and embracing contemporary tools and methodologies.
Innovative approaches such as molecular tools, doubled haploid technology, and refined breeding schemes have greatly contributed to the strides made in CIMMYT’s endeavor to elevate genetic gain within breeding pipelines. These advancements not only drive improved productivity but also promise cost-effective strategies for navigating the challenges posed by climate variability.
Molecular Tools
In maize breeding, traditionally, at each stage of the pipeline, entries are grown in multi-location trials. Phenotyping in multiple environments helps to select the best entries not only based on their genetic values but also on environmental factors and their interaction with diverse environments. However, this is also a labor-intensive and time-consuming step in the breeding pipeline. Molecular breeding offers a transformative solution by expanding breeding programs while minimizing phenotyping requirements. It is a well-known fact that trait phenotype results from both genetic and non-genetic factors, with genetic factors being contributed by the expression of genes at the DNA level.
Identifying genomic regions close to causative genes for traits of interest, such as high yield, disease resistance, or quality, can help to incorporate desirable genes/alleles into selected elite genotypes. DNA-based markers aid in efficiently tracking the inheritance of genetic traits, thereby facilitating the selection of desired traits in breeding programs. Marker-assisted forward breeding accelerates the selection of plants with desired traits by identifying the genetic markers associated with those traits. With such harnessed genotypic information, breeders can pre-select genetic material before embarking on the resource-intensive phenotyping stages. This strategic utilization of molecular markers, particularly in identifying susceptibility to key diseases like maize streak virus (MSV) and maize lethal necrosis (MLN), enables the judicious allocation of resources for phenotyping.

Since 2018, CIMMYT has been implementing marker-assisted forward breeding for MSV and MLN. Since then, more than 100,000 pure breeding lines have been tested by examining their favorable haplotypes with a small set of 10 genetic markers and discarding the lines carrying unfavorable haplotypes for MSV and MLN resistance. In the last six years, nearly 30,000 lines have been rejected before undergoing field testing. In southern Africa, for instance, a rapid response to seed movement using molecular and serological techniques prevented the spread of MLN and facilitated the incorporation of resistance traits into new plant varieties.
Most hybrids in the final stages of breeding pipelines are passed through forward breeding. While Fall Armyworm, Gray Leaf Spot, common rust, and Turcicum Leaf Blight also cause substantial yield reductions in sub-Saharan Africa, research carried out under the AGG project indicates that the genetic makeup of these traits is oligogenic, governed by both moderate and small effect quantitative trait loci (QTLs), but lacking a single major-effect QTL and not amenable to forward breeding. This means that their resistance is influenced by complex multiple genetic factors, rather than being primarily controlled by a few major genetic regions. Alternatively, these biotic stress traits can be improved effectively through genomic selection.
Genomic selection is used to improve complex traits that are controlled by many small-effect QTLs. This approach does not require prior genetic information about the trait of interest and uses genome-wide marker information to estimate all marker effects and select individuals with high genomic-estimated breeding values (GEBVs). This means it uses data from various genetic markers to predict which individuals are likely to have desirable alleles for MSV and MLN. Genomic selection is being applied for grain yield under drought stress, and efforts are underway to extend its application to address more complex challenges related to plant diseases and pests. Foliar diseases are moderately complex traits.
Proof of concept on applying genomic selection for foliar diseases like gray leaf spot and northern corn leaf blight showed high prediction accuracies, supporting the implementation of genomic selection together with forward breeding for other traits at the early stage of the breeding pipeline. Implementing genomic selection for GY under optimum and drought management proved that maize breeders could obtain the same gain as with conventional breeding, where all entries are phenotyped in the field, but at approximately 35-40% less cost. Many candidate hybrids now entering the advanced stages of the breeding pipeline were developed using genomic selection. Several of our earlier studies (Beyene et al., 2015, 2016, 2019, 2021; Chaikam et al., 2019; Crossa et al., 2017; Prasanna et al., 2022; Vivek et al., 2017) showed that breeding pipelines achieved high genetic gain by adopting new molecular tools, thus confirming the benefit of adopting molecular breeding tools.
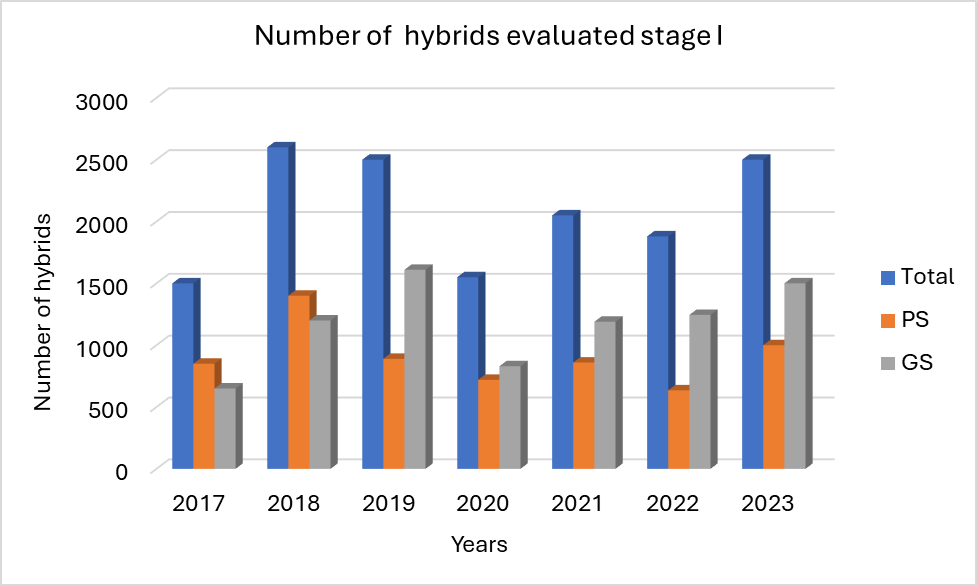
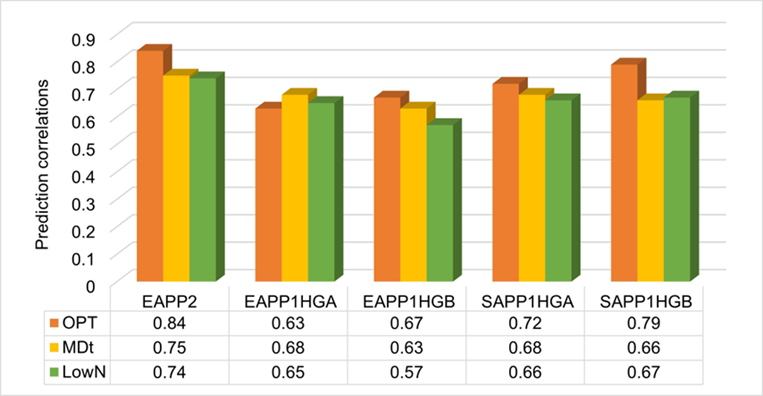
Currently, in CIMMYT’s eastern and southern breeding pipelines, all product profiles are using genomic selection at stage I, where the training population is evaluated in multiple locations with a sparse design, estimating the GEBVs for the unphenotyped lines, and using GEBVs and phenotypic BLUPs of test crosses in the selection for stage II. This process allows the handling of a large number of lines at stage I with a fixed budget without losing selection accuracy. Since 2017, we have used the “test half and predict half” strategy (Figure 2), where all the lines were genotyped with mid-density markers, and the selected ~50% of the total stage I lines were testcrossed and evaluated in multiple locations to be used as a training population to estimate the GEBVs for the other 50% of the unphenotyped lines for the traits of interest. High prediction correlations were observed in three selected product profiles for GY under optimum, managed drought, and low soil N conditions (Figure 3).
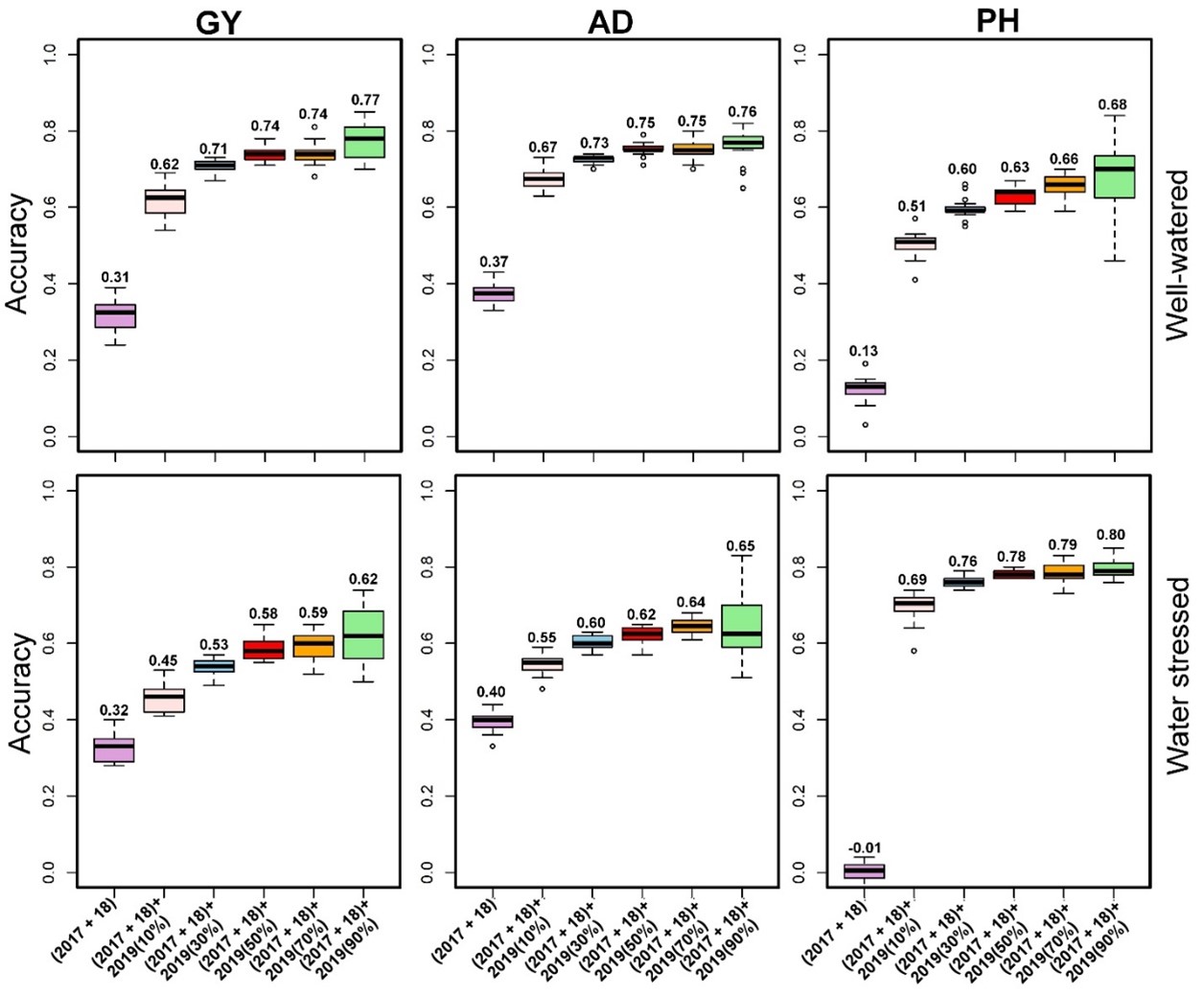
Genomic selection is also implemented to reduce the breeding cycle. However, our final products are three-way cross hybrids, where genomic selection is applied only to select the best line rather than selecting the best hybrid combinations. Historical data were used to test the possibility of reducing the breeding cycle. However, our results showed that the use of historical data to predict 100% of lines from the current year yielded low to moderate prediction correlations both under optimum and drought conditions for GY, anthesis date, and plant height (Figure 4). Incorporating 10 to 30% of the testing population into the training population leads to high prediction correlations. This concludes that by using historical data, the training population, which needs to be test-crossed and evaluated in multiple locations every year, can be reduced from 50% to 10-30%, which helps breeders allocate the saved resources to evaluate more lines without losing prediction accuracy.
Doubled Haploid Technology
Doubled haploid technology speeds up the creation of inbred lines by producing entirely uniform lines. Pedigree line development is a traditional method in plant breeding aimed at gradually improving and stabilizing the genetic makeup of the new variety over time. It involves multiple generations of controlled crosses between parent plants with known characteristics. Each subsequent generation is carefully selected based on specific traits of interest, such as yield, disease resistance, or quality. Pedigree line development is expensive, particularly when nurseries are in remote locations.
Unlike traditional methods where some genetic variation remains, doubled haploid lines are completely homogeneous. This means that there is increased heritability of desirable traits and improved accuracy of selection. Doubled haploid technology, which is more compatible with the use of molecular markers, simplifies breeding processes and shortens the time needed to develop inbred lines (Chaikam et al., 2019).
The first doubled haploid facility in Africa was established in 2013 and is extensively used by the CGIAR, NARES, and the private sector. Over the past five years, 1,349 populations have been induced and more than 223,144 doubled haploid lines delivered to breeding programs from CGIAR, NARES, and the private sector in sub-Saharan Africa. Shifting from traditional pedigree-based breeding to doubled haploid technology has shown a high impact on key breeding metrics (gain per cycle and gain per year) not only in CIMMYT but also in national partners’ breeding programs, thus increasing genetic gain within the available budget.
References
Beyene, Y., Gowda, M., Olsen, M., Robbins, K. R., Pérez-Rodríguez, P., Alvarado, G., Dreher, K., Gao, S. Y., Mugo, S., and Prasanna, B. M. (2019). Empirical comparison of tropical maize hybrids selected through genomic and phenotypic selections. Frontiers in plant science 10, 1502.
Beyene, Y., Gowda, M., Pérez-Rodríguez, P., Olsen, M., Robbins, K. R., Burgueño, J., Prasanna, B. M., and Crossa, J. (2021). Application of genomic selection at the early stage of breeding pipeline in tropical maize. Frontiers in Plant Science 12, 685488.
Beyene, Y., Gowda, M., Suresh, L. M., Mugo, S., Olsen, M., Oikeh, S. O., Juma, C., Tarekegne, A., and Prasanna, B. M. (2017). Genetic analysis of tropical maize inbred lines for resistance to maize lethal necrosis disease. Euphytica 213.
Beyene, Y., Semagn, K., Crossa, J., Mugo, S., Atlin, G. N., Tarekegne, A., et al. (2016). Improving maize grain yield under drought stress and non-stress environments in sub-saharan africa using marker-assisted recurrent selection. Crop Science 56, 344–353. doi: 10.2135/cropsci2015.02.0135
Beyene, Y., Semagn, K., Mugo, S., Tarekegne, A., Babu, R., Meisel, B., Sehabiague, P., Makumbi, D., Magorokosho, C., and Oikeh, S. (2015). Genetic gains in grain yield through genomic selection in eight bi‐parental maize populations under drought stress. Crop Science 55, 154-163.
Chaikam, V., Molenaar, W., Melchinger, A. E., and Prasanna, B. M. (2019). Doubled haploid technology for line development in maize: technical advances and prospects. Theor. Appl. Genet. 132, 3227–3243. doi: 10.1007/s00122-019-03433-x
Crossa, J., Pérez-Rodríguez, P., Cuevas, J., Montesinos-López, O., Jarquín, D., de los Campos, G., et al. (2017). Genomic selection in plant breeding: Methods, models, and perspectives. Trend Plant Sci. 22, 961–975. doi: 10.1016/j.tplants.2017.08.011
Prasanna BM, Burgueño J, Beyene Y, Makumbi D, Asea G, Woyengo V, Tarekegne A, Magorokosho C, Wegary D, Ndhlela T, Zaman-Allah M, Matova PM, Mwansa K, Mashingaidze K, Fato P, Teklewold A, Vivek BS, Zaidi PH, Vinayan MT, Patne N, Rakshit S, Kumar R, Jat SL, Singh SB, Kuchanur PH, Lohithaswa HC, Singh NK, Koirala KB, Ahmed S, San Vicente F, Dhliwayo T, Cairns JE. 2022. Genetic trends in CIMMYT’s tropical maize breeding pipelines. Scientific Reports 12, 20110. https://doi.org/10.1038/s41598-022-24536-4
Vivek, B. S., Krishna, G. K., Vengadessan, V., Babu, R., Zaidi, P. H., Kha, L. Q., et al. (2017). Use of genomic estimated breeding values results in rapid genetic gains for drought tolerance in maize. Plant Genome 10, 1–8. doi: 10.3835/plantgenome2016.07.0070

 Innovations
Innovations